The periodic table didn’t always have 118 elements. Back then, there were gaps like Astatine (Element 85), Francium (Element 87), etc., and the periodic table only ended at Uranium (Element 92). This was because everything on the periodic table only contained natural elements: You could find it on Earth naturally.
Enrico Fermi, a reputable Italian physicist, decided to change that.
An Unfortunate Discovery
Let’s first talk about the basics.
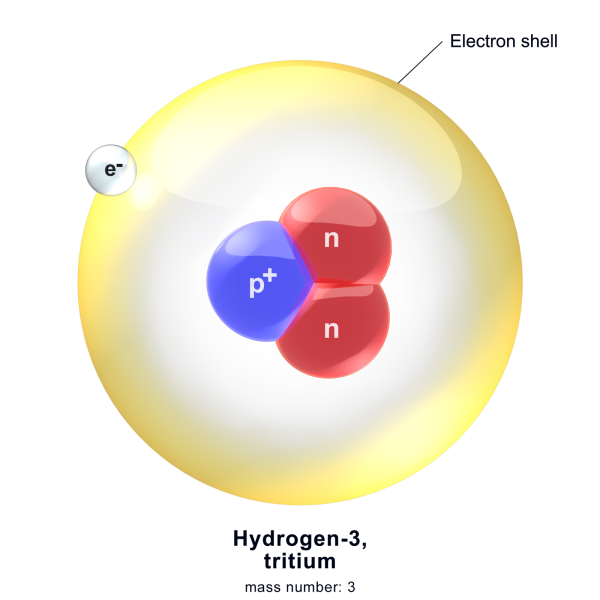
The number of protons an atom has defines what element that atom is. Hydrogen atoms (Element 1) have one proton. Lead atoms (Element 82) have 82 atoms. Neutrons are different. Let’s take Hydrogen-3 as an example. The three at the end describe the total amount of protons and neutrons. Since hydrogen atoms have 1 proton, the remaining two must be neutrons.
Nuclides are more specific than elements. They describe the combination of protons and neutrons in an atom. Hydrogen-3 is a nuclide as it comprises a proton and two neutrons. Some nuclides can remain unchanged for billions of years, while others decay in milliseconds.
Hydrogen-3 is radioactive, so one of its protons decays into a neutron, releasing an electron (and neutrino). The result is Helium-3. This process is called beta minus decay.
Fermi used beta minus decay to his advantage. He could start with Uranium (Element 92) and use beta minus decay to add one more proton, creating element 93. He fired neutrons at the Uranium to hopefully start the process.

It worked! He discovered elements 93 and 94 and named them Ausonium and Hesperium. But when another lab recreated Fermi’s experiment, they found that he had done the unthinkable: He had split the atom. Splitting an atom releases huge amounts of energy, and this powerful reaction paved the way for the invention of nuclear weapons.
Journey Begins
The University of California, Berkeley, decided to try it. They used the cyclotron, a particle accelerator on a smaller scale. In 1940, they discovered element 93 and gave it the official name Neptunium.
Glenn Seaborg, an American scientist, took the spotlight. Working with other members at Berkeley, they discovered element 94: Plutonium. This element was used in the Manhattan Project as a better alternative to Uranium. The university would go on a spree from here, finding elements 95 to 98.
Elements 99 and 100 were discovered differently. A nuke was dropped in the Pacific Ocean, and samples of the mushroom cloud were collected. What they observed were two new elements: Einsteinium and Fermium. Element 101 was quickly found afterward and called Mendelevium, named after Dmitri Mendeleev, who is credited with the periodic table you see today.
Glenn Seaborg is credited with most of these discoveries today.
New Opponents
A new lab in the USSR would try to one-up Berkeley. The Joint Institute for Nuclear Research (JINR) would claim elements 102 to 105. So did Berkeley. Both argued about who discovered the element and who had better data. The race ended with element 106, as Berkeley had a new opponent to worry about.
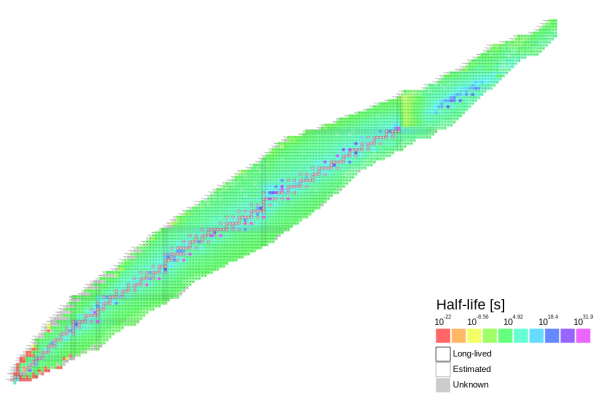
There’s a growing problem as you go up the nuclide table. Most nuclides past Uranium (Element 92) are radioactive due to their instability. When finding new elements, this can be a problem because alpha decay can happen in hours, minutes, or even seconds. Alpha decay is when an unstable nuclide spews 2 protons and neutrons out.
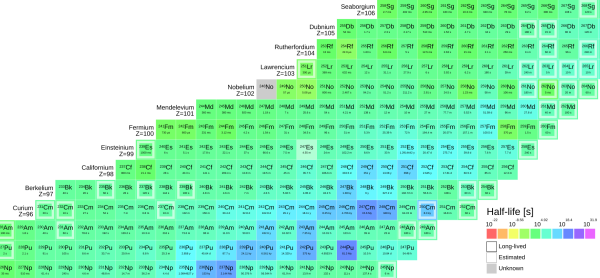
Mendelevium is where alpha decay gets so bad that only one atom can be made at a time. Once another Mendelevium atom is made, the other decays.
A new method, though, could avoid this. On the nuclide table, nuclides with a certain number of protons and/or neutrons seemed highly stable. The next predicted stable numbers would be 114 protons and 184 neutrons. If scientists could skip eight elements to get there, atoms would be at least somewhat stable. Then, element 114 would alpha decay to discover elements 112, 110, and 108.
Then, instead of launching neutrons to create beta minus decay, you could launch heavier atoms like Iron (Element 26) onto Lead (Element 82). This required new technology but was worth it. GSI, in Darmstadt, Germany, had the technology. GSI had discovered elements 107 to 109. For the first time in decades, Berkeley was behind.
The JINR had returned and partnered with the Lawrence Livermore National Laboratory (LLNL) in Livermore, California. They made the jump to element 114. Meanwhile, GSI had discovered elements 110 to 112 in just months.
Making a Comeback
Victor Ninov was hired by Berkeley in hopes of making a comeback. They were going to jump to element 118. So, the lab members set everything up and left for vacation while Ninov stayed in the lab. Not even a month later, Ninov told everyone that he had discovered element 118, as well as 116 and 114, due to alpha decay.
For some reason, he was hesitant to show the results.
When GSI recreated the experiment, there was no element 118. The same was true for the alpha decay chain. Other labs around the world recreated the experiment, too. The same thing happened. Ninov had no clue what had happened and refused to speak about it.
Ninov had made Goosy, a program that analyzed nuclides. Now, it was thought that this program had made a mistake. But when files were investigated, it was clear that the results were fake. Someone had tampered with the files, and further investigation showed that Ninov had made the edits. He was eventually fired.
In 2002, element 118 was discovered and named Oganesson. This time, it was confirmed.
“The education of young people in science is at least as important, maybe more so, than the research itself.” – Glenn Seaborg
RELATED STORIES:
https://bigthink.com/the-past/uc-berkeley-ninov-elements/
https://phys.org/news/2016-01-elements-periodic-table.html#google_vignette
https://www.acs.org/education/whatischemistry/landmarks/transuranium-elements-at-berkeley-lab.html
TAKE ACTION:
- Visit the nuclide table website: https://people.physics.anu.edu.au/~ecs103/chart/